Over the summer we filed a Freedom of Information Request with the US Food and Drug Administration to obtain the toxicology studies filed by the manufacturer of Tinuvin® 770 in order to get it approved to be an additive in adhesives and pharmaceutical-grade plastics. Most of the documents are from 1975-76.
We received the tranche of documents on September 10, 2024. While almost all are animal studies, there is way more information than in the academic literature. (Which is why we always caution against drawing inferences from what little leaks from industry to scientific journals.) We are posting all the documents we received here without comment so our community can learn from them.
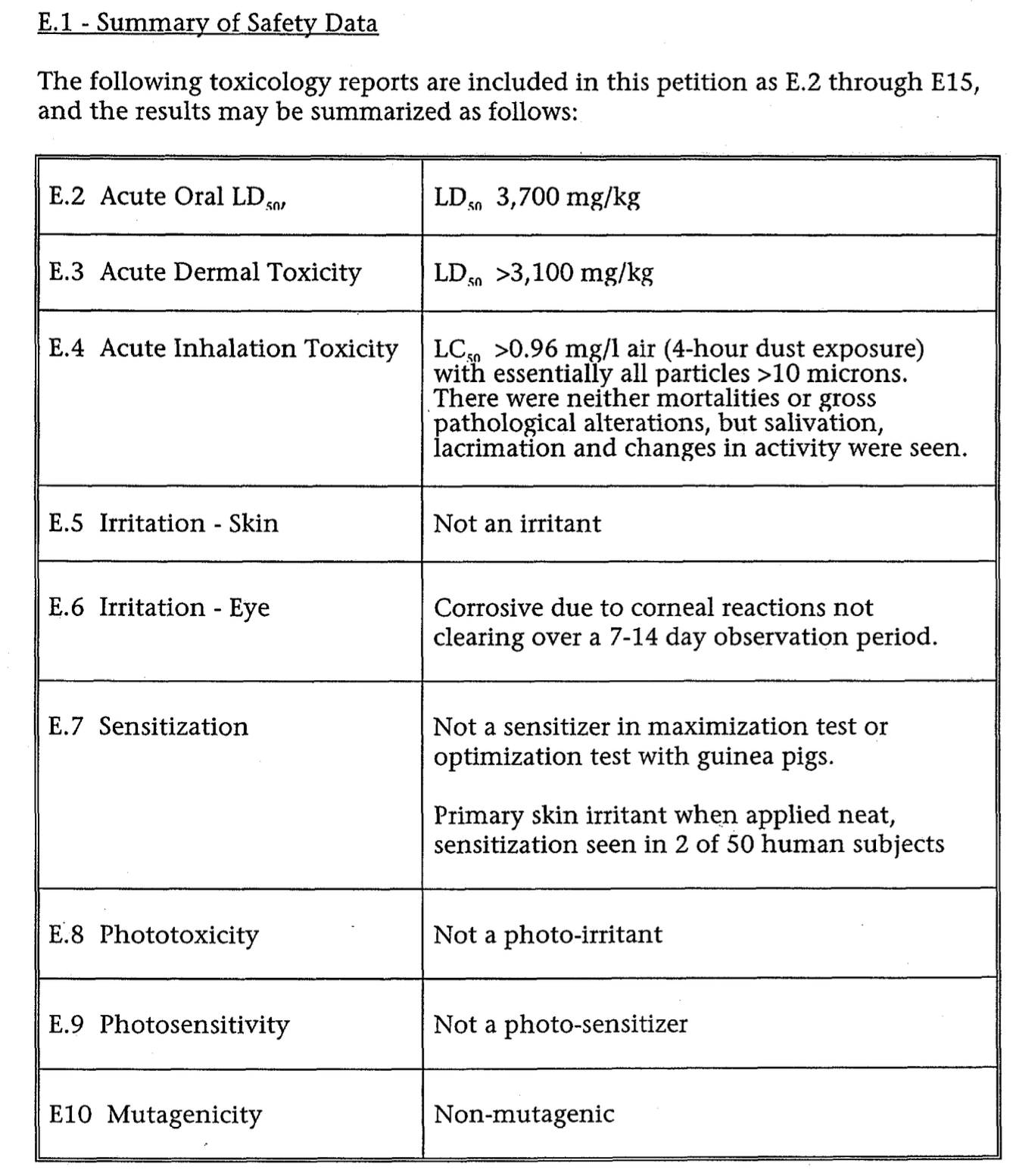
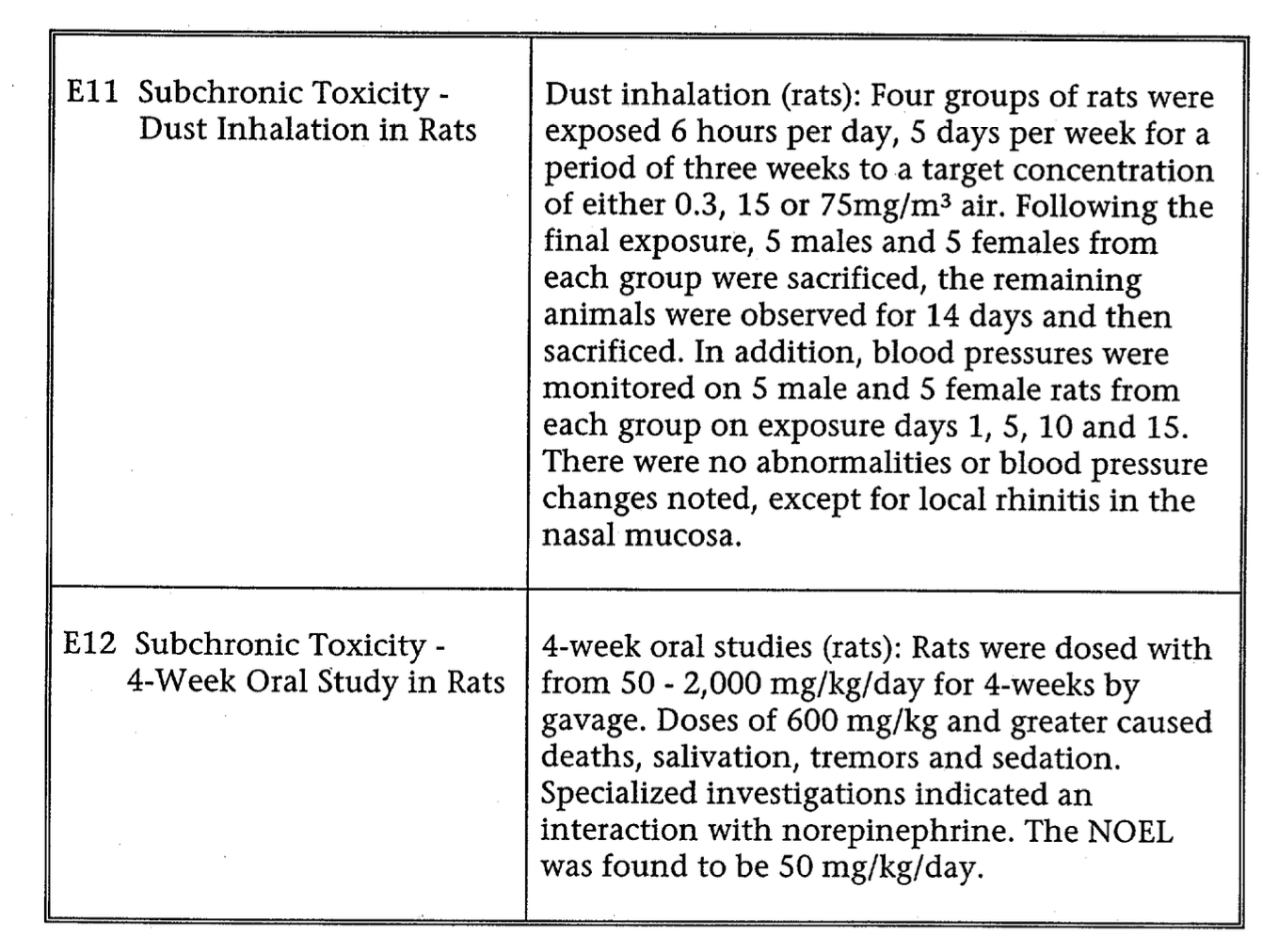
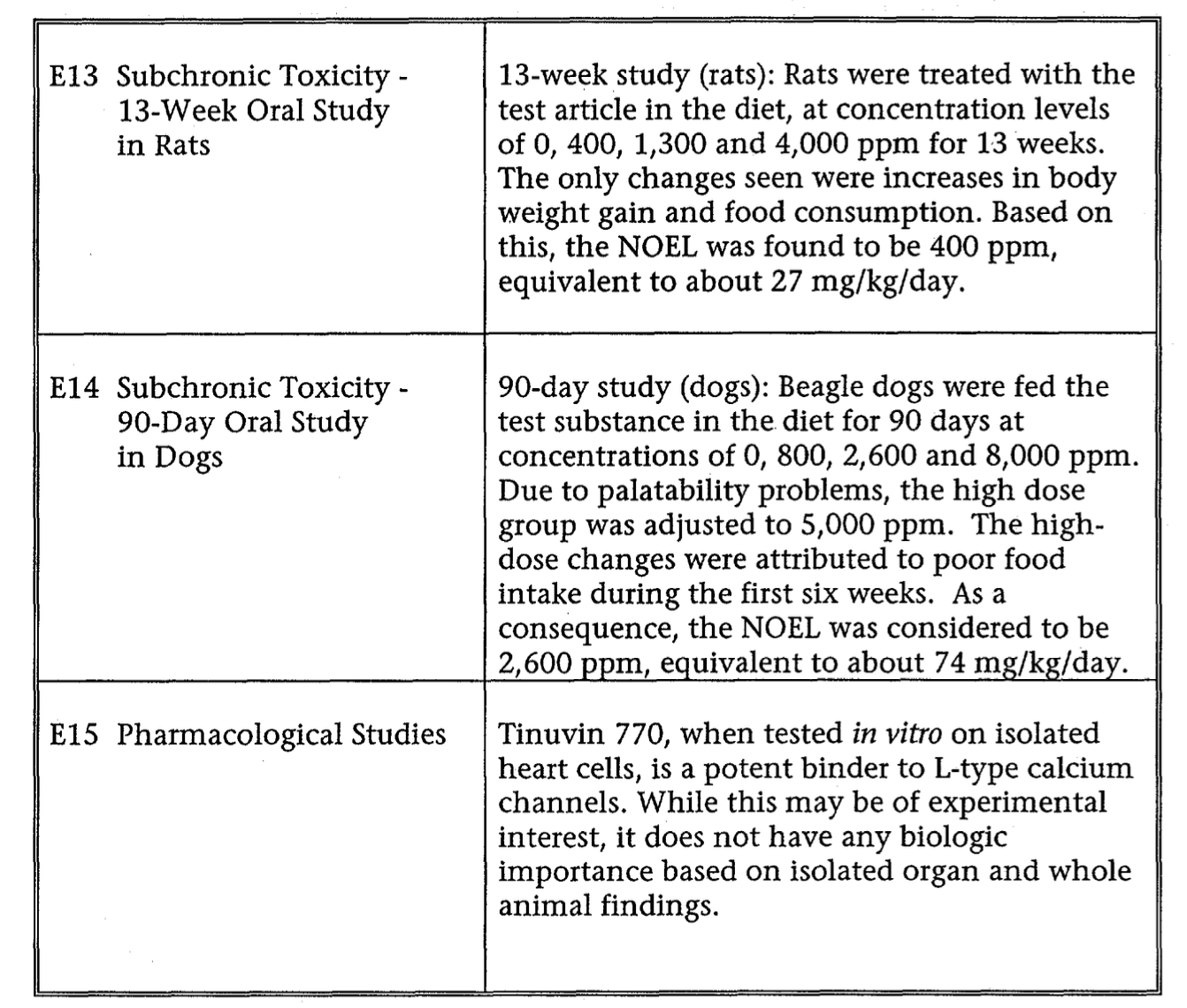
Summary of Safety
This is the Summary of Safety information submitted by the manufacturer in 1976. These tables summarize the the studies in PDFs that follow.
Cardiac Tissue
This was as a surprising conclusion to me:

Specific Toxicity Studies
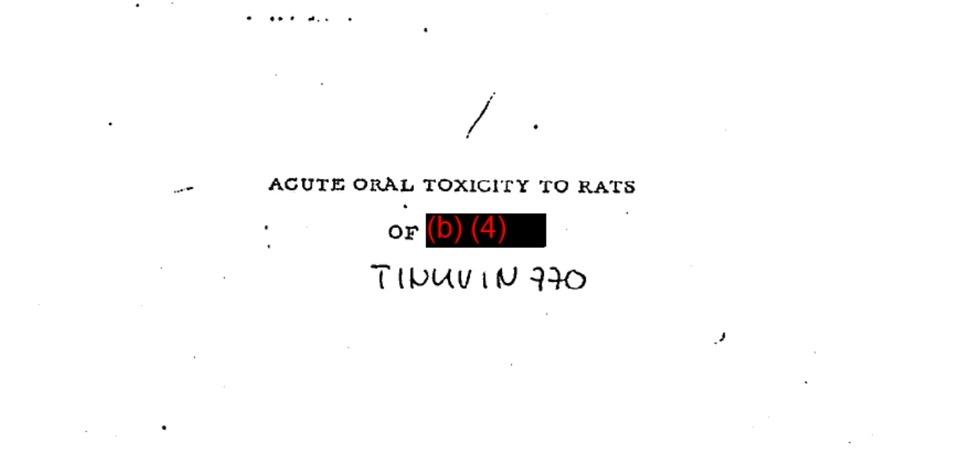
Acute Oral Toxicity to Rats
Acute Dermal LD50 in Rat
Acute Dust Inhalation Toxicity and Acute Vapor Inhalation Toxicity Society
Acute Vapor Inhalation Toxicity in Albino Rats
Acute Dust Toxicity
Acute Vapor Inhalation Toxicity
Skin Irritation, Sensitization, Phototoxicity
Acute Eye Irritation
Human Skin "Repeated Insult"
Salmonella/Mammalian Mutagenicity
Dietary Toxicity
FDA Review as Food Adhesive
Review of studies by FDA in August 1998 for the use of Tinuvin® 770 as an adhesive for food.